Longevity Biotech is currently developing LBT-3627 as a novel neuroprotective agent as a possible treatment for disorders such as Parkinson's Disease. LBT-3627 is a Hybridtide® that targets the VIP family of receptors - specifically the VPAC2 receptor subtype. Empirically, VPAC2 provides more protection than the closely related VPAC1 receptor. Together with the team at the University of Nebraska Medical Center, we have worked to determine the details regarding the protective mechanism along with functional endpoints. The results were recently published and describe a variety of experiments each providing insight into the unique immunomodulatory mechanism of action of LBT-3627. Longevity Biotech is actively progressing this program through preclinical development and towards clinical trials. Our initial focus is on neurodegenerative disorders, including Parkinson's Disease.
LBT-3627 was specifically designed to be >1000x selective for VPAC2 over the closely related VPAC1. Selectivity was determined using engineered mammalian cell lines with the human receptors, using cyclic AMP (cAMP) as the readout for GPCR activation. LBT-3627 was then further optimized for protease stability resulting in the most selective and most stable VPAC2 agonist available.
CLICK HERE TO DOWNLOAD SLIDES
VIP reduces Neuroinflammation
Vasoactive Intestinal Peptide (VIP) is a well known
anti-inflammatory agent that was discovered nearly 40 years ago.
Peripheral Immunomodulation
The VIP pathway is an well-established anti-inflammatory pathway that is upstream of many key inflammatory targets.
Pro vs. Anti
The VIP pathway is involved in the rebalancing TH1 / TH2 / TH17 cytokine profiles. Specifically, LBT-3627 down-regulates pro-inflammatory INF-γ while up-regulating anti-inflammatory IL-10.
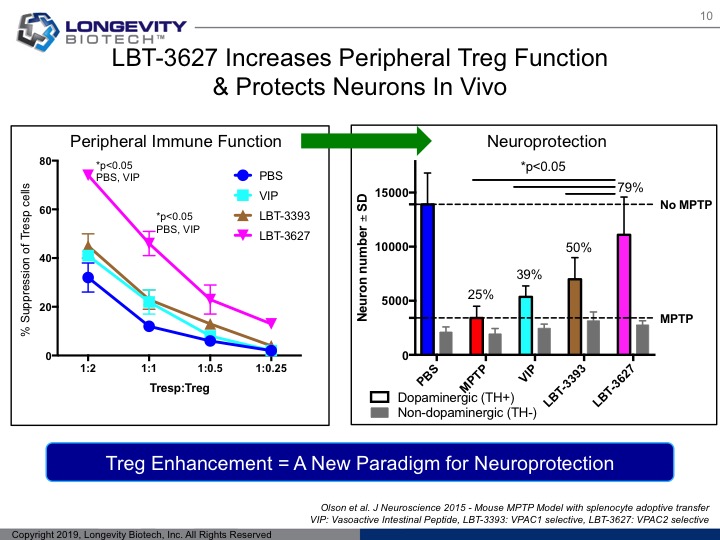
In vivo neuroprotection
LBT-3627 has demonstrated nearly 80% protection of TH+ dopaminergic neurons via MPTP neurotoxic models in vivo.
By favorably shifting the peripheral immune system to an protective profile, LBT-3627 has demonstrated robust neuroprotection using in vivo neurotoxicity models.